Wednesday, May 22, 2019
This is very big news.
This is the first non-chemotherapy based fixed duration treatment for CLL in treatment naïve patients.
Until this approval, the only viable approved treatments were fixed duration chemotherapy or taking ibrutinib until progression or side effects force you off.
Of course, venetoclax and obinutzumab could have been and have been used together “off label” as they are both already approved medications, but now insurance will have no excuse not to pay for it. And doctors less familiar with treating CLL should feel comfortable with an another strong option for a first therapy.
The Phase III study was done by the well-respected German CLL study group lead by Prof. Hallek out of Cologne.
Here is a link to the Abbvie press release.
Below is part of the press release from Genentech who is co-developing venetoclax with Abbvie
About the CLL14 Study
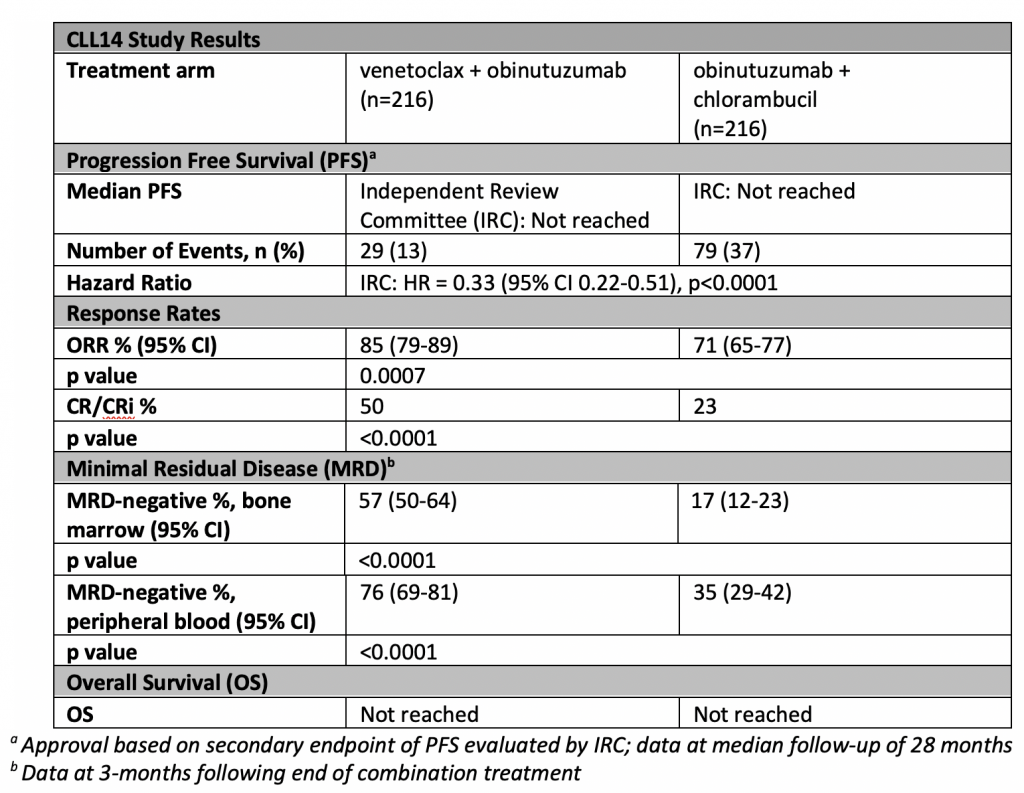
CLL14 (NCT02242942) is a randomized Phase III study evaluating the combination of fixed-duration venetoclax plus obinutuzumab compared to obinutuzumab plus chlorambucil in patients with previously untreated chronic lymphocytic leukemia (CLL) and co-existing medical conditions. 432 patients with previously untreated CLL were randomly assigned to receive either a 12-month duration of Venetoclax alongside six-month duration of Obinutuzumab (Arm A) or six-month duration of obinutuzumab plus chlorambucil followed by an additional six-month duration of chlorambucil (Arm B). Arm A started with an initial cycle of obinutuzumab followed by a five-week venetoclax dose ramp-up to help reduce tumor burden. The primary endpoint of the study is investigator-assessed progression-free survival (PFS). Secondary endpoints include PFS assessed by Independent Review Committee (IRC), minimal residual disease (MRD) status, overall response (OR), complete response (with or without complete blood count recovery, CR/CRi), overall survival (OS), duration of response (DOR), event-free survival (EFS), time to next CLL treatment (TTNT), and safety. The CLL14 study is being conducted in cooperation with the German CLL Study Group (GCLLSG), headed by Michael Hallek, M.D., University of Cologne.
The most common adverse reactions with Venetoclax plus Obinutuzumab were low white blood cell count (neutropenia), diarrhea, fatigue, nausea, low red blood cell count (anemia), and upper respiratory tract infection.
Comments:
This is a powerful new option for patients to consider. Just how durable these responses will be remains to be determined, but the depth of the remissions with ¾ of patients reaching U-MRD (undetectable minimal disease or less than one CLL cell per 10,000 white blood cells) in the peripheral blood and 56% in the bone marrow bodes well for lengthy responses. For more on MRD from Prof. Hallek , see this interview from ASH 2017. What we patients want to see if very durable PFS (progression fee survival) and OS (overall survival), and the early results are promising.
Moreover, hopefully this is the first of many such approvals of fixed duration combinations that will be coming over the next few years that could revolutionize how CLL is treated. And of course, the approval raises the question as to which combos and sequences are best.
Exciting times for us patients.
Brian Koffman, Chief Medical Officer, CLL Society
Sunday, May 12, 2019
Acalabrutinib Phase III ASCEND trial met primary endpoint at interim analysis in relapsed or refractory chronic lymphocytic leukemia and will stop early
This trial in previously treated CLL patients showed a clinically significant progression free survival when compared to a combination regimen of rituximab plus physician’s choice of idelalisib or bendamustine. No new side effects or problems were noted.
While not unexpected, it is important to have this trial confirmation that another BTK inhibitor, like ibrutinib has proven to save lives.
This is more good news for CLL patients. Acalabrutinib is already approved for mantle cell lymphoma (MCL) and hopefully will be soon approved for us CLL patients based on this and Phase 3 trials that should have positive results soon.
While not approved for CLL, because it is approved for MCL and is part of the NCCN guidelines for CLL, it is already an option today for appropriate CLL patients if your doctor pushes for it.
Here is a link to a nice review of the data we have so far and more trial news.
Here is the official press release.
It is good to have more choices of all these great drugs. Can’t wait for approval to make it easier to access.
Stay strong,
Brian
Brian Koffman MDCM DCFP, DABFM, MS Ed
Co-Founder, Executive VP and Chief Medical Officer
CLL Society, Inc.
Co-Founder, Executive VP and Chief Medical Officer
CLL Society, Inc.